Coflore slurry reactor: Hydrogenation with Hydregen
- Jun 7, 2023
- 4 min read
Catalytic hydrogenation reactions are important in the chemical industry, with the presence of a catalyst such as nickel, platinum or other material typically required for the reaction to complete.
There are two types of hydrogenation reactions, heterogeneous and homogenous. Heterogeneous metal-catalysed hydrogenation processes are widely used because of their efficient atom use and recyclable catalyst. The catalyst is often a solid, which makes it relatively easy to recover and recycle.
Continuous flow has proven successful at adopting hydrogenation reactions, with improved gas-liquid mass transfer rates and catalyst continuity, making continuous flow a more established process than batch. The smaller operating volume of hydrogen gas also makes flow chemistry a safer alternative. However, there is still room for improvement in hydrogenation flow reactions, from reducing the reliance on toxic and expensive precious metals to minimising the energy-demanding conditions and flammable solvent use.
It is this opportunity that led AM Technology to work with HydRegen. Their aligning ethos, to create a cleaner, safer and more efficient chemical manufacturing approach, led to us collaboratively working together to introduce a biocatalyst to the continuous flow hydrogenation reaction.
This only fuelled HydRegen’s mission to embed bioprocesses in the industrial sector whilst propelling AM Technology’s aim of pivoting flow chemistry as the realistic alternative to batch chemical manufacturing.
Embedding Hydregen’s bio-process approach at industrial R&D level
A heterogeneous biocatalytic hydrogenation is an alternative strategy using H2 gas and biocatalysts, which are safer and more sustainable than the precious metals typically used in catalysis.
HydRegen and AM Technology have been working together to introduce a bio-based manufacturing approach to flow chemistry, with our recent publication discussing the experiments designed to test whether a biocatalytic hydrogenation approach can be used in a continuous hydrogenation reactor and how it compares to metal-catalyzed processes.
The case study reaction focused on reducing 3-quinuclidinone to (3R)-quinuclidinol, a key component in approved antimuscarinic drugs.
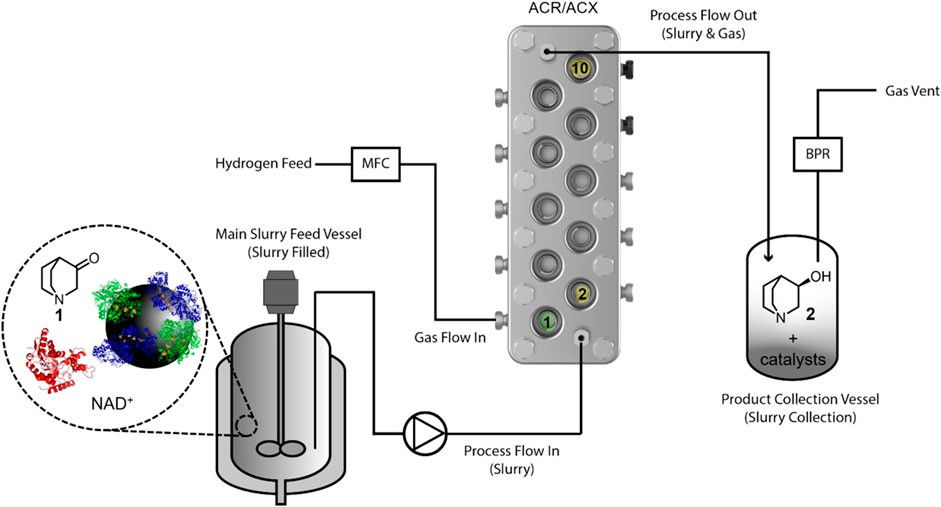
The study used the Coflore ACR, developed by AM Technology, a dynamically mixed continuous flow reactor that is ideal for early-stage industrial R&D. Our publication discusses the translation of the biocatalytic hydrogenation process from small-scale batch reactions to the Coflore ACR flow reactor. Learn more about how we teamed up with HydRegen to tackle the project at each phase below:
Or read the full publication here.
Selecting suitable carbon material for the translation of biocatalytic hydrogenation into Coflore reactors
HydRegen tested different types of carbon materials to see which ones would work best to support the biocatalyst and found that carbon black worked better than activated charcoal at adsorbing the enzymes. They also tested to see if the enzymes would leak out of the carbon after being adsorbed and found that there was minimal leaching. They concluded that a carbon black slurry process would be the best way to move forward with their research.
The slurry handling capabilities of our Coflore reactors is something that we’ve been passionate about at AM Technology. About half of chemical processes are heterogeneous, and with many chemical processes featuring suspended solids, the hydrogenation reaction is no exception. Hydrogenations account for about 15% of all industrial chemical processes.
Phase one of this collaborative research was to ensure we had identified the best way to use HydRegen’s biocatalyst in a slurry reactor. To do this, various types of carbon materials were tested to see which would best support the biocatalyst.
Scale-down batch reactions
In this experiment, one type of biocatalyst was used to convert 3-quinuclidinone into (3R)-quinuclidinol using hydrogen gas. HydRegen tested how well the catalyst worked under various conditions, including temperature and catalyst concentration.
When comparing to commonly used metal catalysts, it was confirmed that the biocatalyst was more efficient and completed the reaction in a shorter overall time.
Biocatalysed hydrogenation of quinuclidinone in a continuous flow slurry reactor
One difference between biocatalytic and metal-catalysed hydrogenation, is the solvent use. Biocatalytic hydrogenation requires aqueous media, however this has implications for H2 solubility. When performing the biocatalytic hydrogenation of 3-quinuclidinone, the use of the Coflore ACR was expected to overcome the rate limitation of hydrogen dissolution into the solvent which would open opportunities to use aqueous solvent.
The increased mass transfer of the Coflore ACR, AM Technology’s laboratory scale flow reactor, showed results of the biocatalytic hydrogenation of 3-quinuclidinone achieving conversions up to 100% with no issues observed after extended operation.
Enabling the use of water instead of toxic, flammable organic solvents was a desirable outcome as it lowers overall health and safety risks of a hydrogenation process. This also aligns with one of the 12 principles of green chemistry (Principle 5, safer solvents) which recommends minimising or avoiding the use of auxiliary substances like solvents or using safer alternatives whenever possible.
Using Pd/C, to convert 3-quinuclidinone into 3-quinuclidinol
Collaboratively, we executed an experiment that involved using a chemical reaction to convert 3-quinuclidinone into 3-quinuclidinol, using Pd/C. The experiment was carried out under high pressure conditions (10 bar H2), which required different reactor equipment to be used compared to previous experiments. Specifically, a different pump and a catalyst basket agitator were used to simplify post-reactor processing through catalyst immobilisation within the reactor cell block, avoiding the need for catalyst filtration and recirculation which are required with a slurry-fed catalyst.
The experiment was carried out twice, with different amounts of Pd/C catalyst used. In the first experiment, a high amount of catalyst was used, which resulted in full conversion of 3-quinuclidinone to 3-quinuclidinol , but with low Pd turnover numbers.
In the second experiment, less catalyst was used with a higher concentration of 3-quinuclidinone used to compensate for this. The results showed that the catalytic activity was similar to a previous experiment carried out at lower pressure and with a smaller amount of catalyst. The experiment also produced a mixture of both enantiomers of 3-quinuclidinol, as revealed by chiral-phase GC-FID analysis. This is not a desirable outcome for the application toward pharma, though is typical with Pd/C catalytic hydrogenation.
Conclusion
The team of scientists involved in this project, including AM Technology’s own, have developed a new method for converting ketones to (3R)-quinuclidinol using a biocatalytic hydrogenation process used in continuous flow reactors
.
This biocatalytic system operates at mild conditions, achieving high conversion rates without requiring additional optimisation. It can replace traditional metal catalysts in hydrogenation reactions, leading to potential benefits in energy efficiency and safety while reducing carbon emissions.
This new process uses enzymes that work well under mild conditions and can be used in place of harmful chemicals. It can also be used at higher concentrations, which may lead to even more efficient reactions.
Project scientists:
Flow Chemist, Martin Monedero
Business Development Manager, Michael Kenny
Chemical Engineer, Joshua Trenchard









Comments